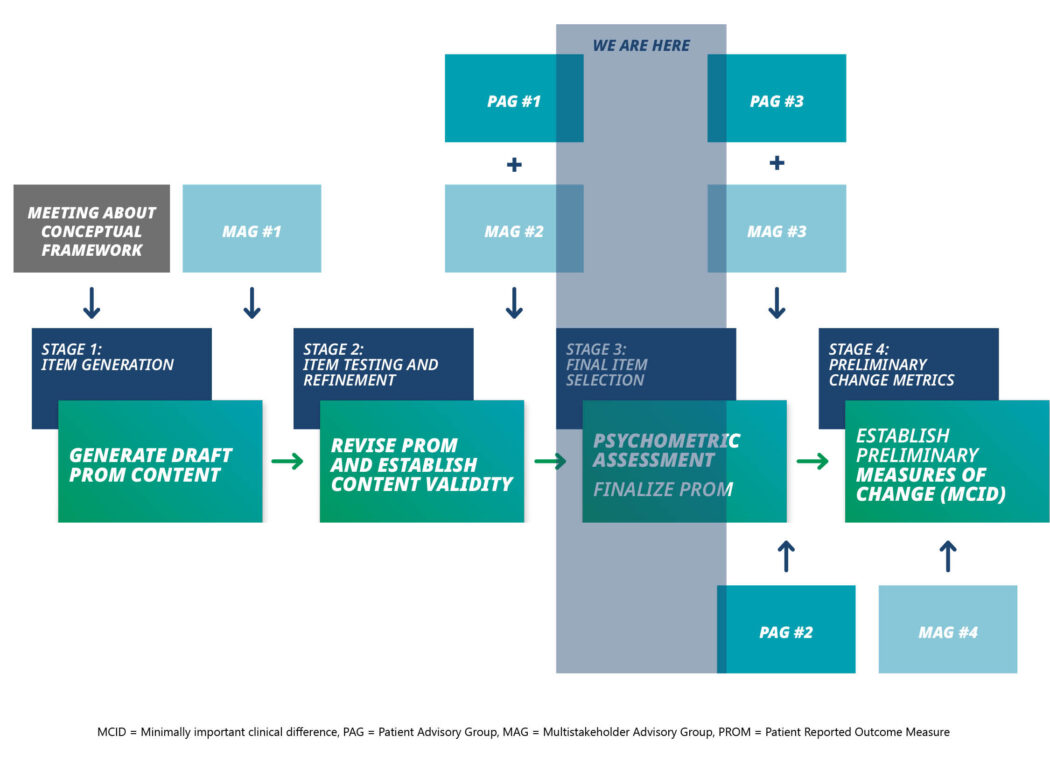
To meet the aim of the study we will complete all four stages of the study, by completing all 10 of the key study objectives contained within these.
CLINICAL TRIAL REGULATION TIMELINE
Item Generation
There are 2 main purposes for Stage 1:
- Drawing on the content, data, and analysis from a previous international patient online survey in iMCD, generate draft PROM content (including a longlist of candidate draft items under existing themes) to assess symptom burden in iMCD.
- Incorporate expert opinion and lived experience to achieve consensus on which items will be tested for inclusion in the symptom burden scale via an online multistakeholder group (MAG) workshop involving the funder, research team, patient and public involvement and engagement (Patient Advisory Group – PAG) representative(s) (i.e., patients with iMCD and/or their carers), healthcare professionals, and any other relevant stakeholders.
Item testing and refinement
- In cognitive debriefing interviews with people living with iMCD, assess the content validity of the items (and associated PROM content) in terms of relevance, comprehensibility, and comprehensiveness. This will include assessing whether they are important/meaningful to patients.
- Analyze and summarize qualitative evidence on content validity, make recommendations on revisions to the draft PROM, and agree this with the multi-stakeholder advisory group (MAG; as described above) and a separate group of PAG collaborators.
Final item selection
- Administer the refined PROM (from Stage 2) to as wide a sample as possible of people living with iMCD. The PROM will be administered alongside additional sociodemographic and clinical questions and measures of burden and/or health-related quality of life (HRQoL).
- Taking into account the sample size, conduct appropriate psychometric analyses in order to provide evidence for final item selection and to provide preliminary psychometric evidence on the reliability and validity of the final measure.
- Taking into account all of the available qualitative and quantitative evidence, and a consultation with PAG collaborators, agree (with the MAG) on final item selection, with the goal of obtaining a concise (e.g., 8-10 item) measure of symptom burden.
- Preliminary measures of change: Re-administer the refined PROM (from Stage 2) to participants (from Stage 3) alongside measure(s) of perceived clinical change
- Conduct qualitative interviews with participants who have completed the PROM at both time points to understand what constitutes a meaningful difference from their perspective.
- Triangulate quantitative and qualitative data to estimate a minimally important clinical difference (MCID) for the new PROM, agreed with the MAG.